HVAC Design for USP 797 & 800 Cleanrooms
Last month, we touched on the current status of revisions to USP General Chapters <795> (Pharmaceutical Compounding – Non-Sterile Preparations) and <797> (Pharmaceutical Compounding – Sterile Preparations) and their overall affect on the compendial status of USP <800> (Safe Handling of Hazardous Drugs). To catch up, check out the article below:
As its stated in the article, its likely that the revisions will go in effect sometime next year, still giving pharmacy facilities to make the appropriate upgrades to meet USP <797> and <800>. For those pharmacy facilities who do require new upgrades to comply with the standards, you have options that must be addressed to ensure a compliant, yet functional, environment can be constructed. The best option will depend on the variables of the facility and cleanroom required based on intended operations.
Where To Start?
A highly recommended first step, prior to diving into the planning stage, is to contact your respective State Board of Pharmacy. Each state has unique requirements and interpretations for USP <800> compliance and will save you time during to Design Phase knowing those requirements on the front end.
Design Benchmarks
Design standards in USP <797> and <800> affecting the HVAC design of the pharmacy include:
HVAC Concerns
Not only are healthcare renovations inherently complicated. Maintenance of operations, infection control, and unknown existing conditions contribute to this complexity. A pharmacy renovation for USP <797>/<800> compliance involves said items, plus the additional technical challenges associated with higher levels of air filtration, higher air change rates, differential pressure, and additional exhaust requirements. The project team, including the facility pharmacist, architect, cleanroom vendor, and HVAC engineer, must be involved in planning the compounding area. Identification of critical area type and equipment placement is important to HVAC design and air distribution.
Pressure relationships, airflow, and cleanliness are vital to maintaining the cleanroom condition required in each space. Hazardous drug (chemo) prep rooms under USP <800> are required to have a negative pressure of .01 to .03 in. water column to all adjacent areas and for the room(s) to be externally vented via exhaust. Air that would normally be recirculated into the air handling system is now less available. The system must accommodate greater volumes of make-up air, and thus also provide the extra capacity for heating and air conditioning. Not providing enough air to the cleanroom may trip alarms, create a pressure differential that is too negative, or cause failures and burn out within fan drives.
Cleanrooms with non-hazardous drug preparation are required to have a minimal positive pressure differential relationships of 0.02 in. water column to their anteroom, which also is positive to the corridor or adjacent work space via a cascading effect.
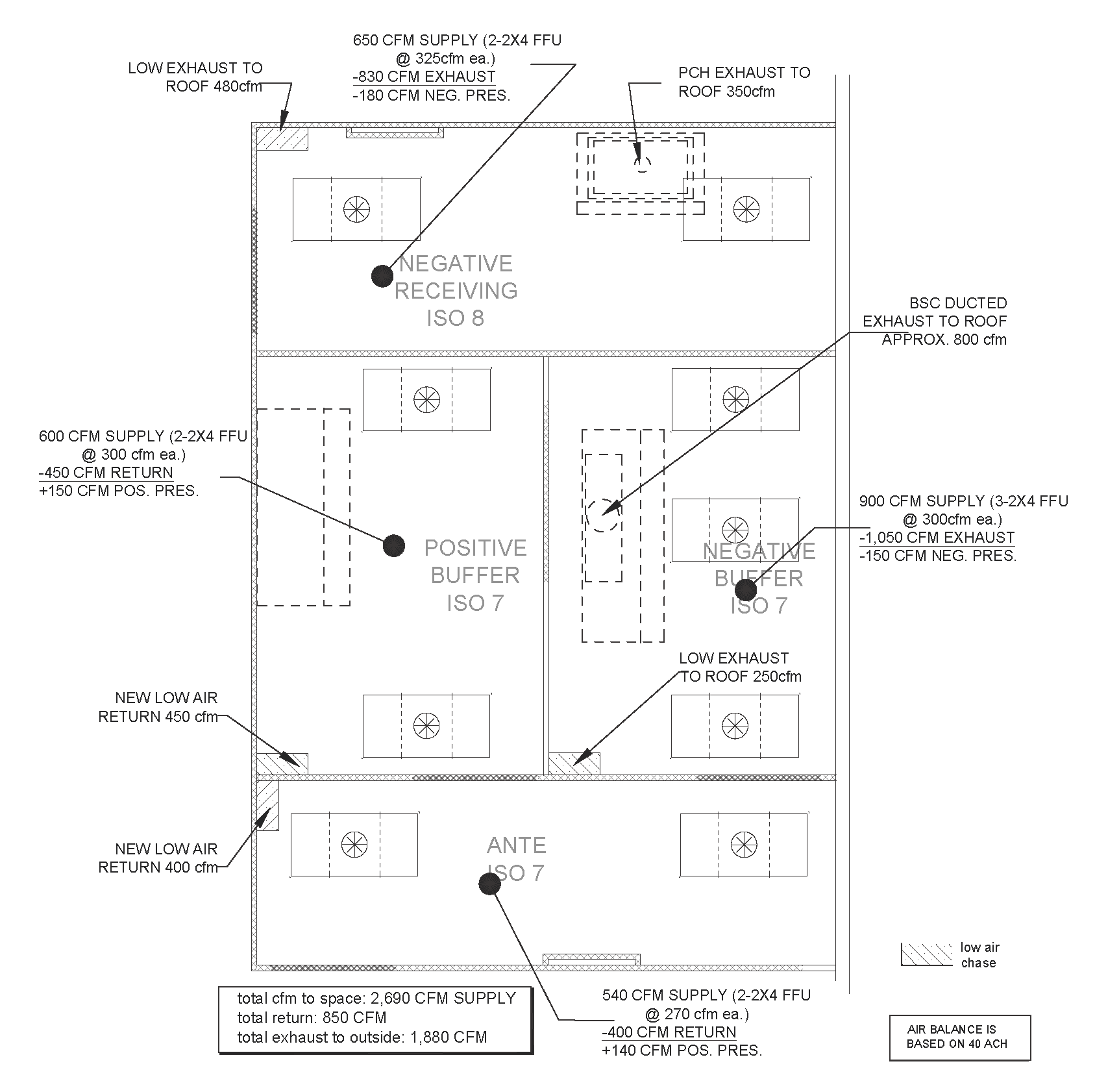
Specific airflow problems can exist in the design of the space, which can cause major headaches for the design engineer. Below, a mock air balance diagram, shows the required supply air to feed into a 4-room cleanroom complex (included HD Receiving Space) as well the required exhaust for the negative pressured rooms to all be balanced based on achieving 40 air changes per hour. Each space we account for number of HEPA units, the specific PEC and any/all air returns/exhausts. Many rooms have two or more hoods to account, and above-ceiling coordination can be an issue that needs addressed.
In many cases, an existing air handling unit shared among the outer facility spaces cannot feed sufficient air to the cleanroom suite that includes hazardous compounding room while maintaining required temperatures, humidity and air changes. It is best recommended that a dedicated air handler be provided to independently control the cleanroom suite to ensure temperature, humidity, air changes per hour and pressure differential are maintained at all times and throughout the changes in season.
Where upgrades to the current air conditioning system are not an option, sometimes in-line supply air fans are needed to overcome the added static pressure and dedicated 100% outside-air air conditioning units are needed for proper space conditioning and air supply.
Advantages of Variable Speed HEPA Motors for USP Compliant Cleanrooms
The cleanroom suite will require rebalancing to validate adequate pressure differentials. Upgrading your HEPA fan filter system to remote variable speed motors is an efficient way to balance differential pressure. A variable speed fan filter system allows balancing of initial pressure calibration, but is also robust enough to overcome filter load throughout the life of the HEPA filter. Advantages include:
Fan speeds are set and/or adjusted from a console located outside the cleanroom without breaching the integrity of the cleanroom.
Room or equipment layouts with unique airflow requirements are easy to set and control.
Variable fan speed controllers reduce overall fan filter maintenance costs. Static drive fan filter units will require more frequent HEPA filter replacement because fan is speed is not adjustable to overcome filter blockage.
Solution?
It’s beneficial to understand your specific requirements based on your state regulatory inspectors. Once the end-user has defined the need for C-PEC and C-SEC based on intended operations/products, the systems and space can be properly designed among all working groups. Knowing the existing HVAC system design parameters during renovation also will provide insight into the airflows available to assist in system design and space pressurization.
ProPharma Cleanrooms initial consultation and assessment (provided at no charge) guides pharmacy and facilities team members toward compliance specification, design components and construction variables to ensure a fully functional and compliant cleanroom environment.
About ProPharma Cleanrooms
Serving health system pharmacies, 503A Compounding Pharmacies, FDA registered 503B Outsourcing Facilities and Manufacturing sites across the nation; ProPharma Cleanrooms turn-key services and cleanroom components are best-in-class. Because we take pride in our builds, ProPharma Cleanrooms takes the necessary steps to microbially clean each ISO rated space to prepare for certification and train the pharmacy staff on use an maintenance for long term sustainability. For help with a cleanroom construction project, please contact a representative today.